Scientists discover the reason that batteries lose capacity over time: Nanocrystals!
We marvel when a new smartphone crams in a few more milliamp-hours. Capacity is nothing without the longevity to survive a large number of repeated charging cycles, though. Even the most advanced lithium-ion batteries still lose capacity as they age, and there’s no way to prevent that until we know the cause, which we might thanks to two new studies from the US Department of Energy. These studies point to tiny nanoscale crystals as the culprits for reduced capacity over time.
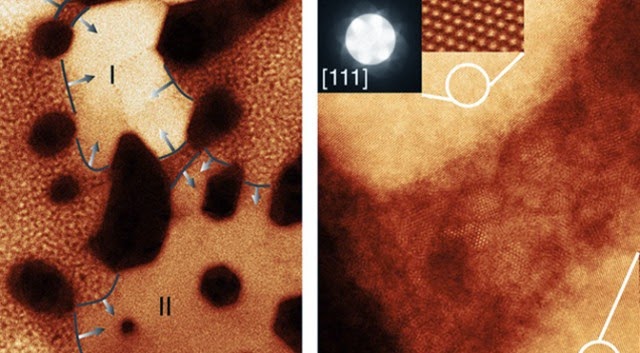
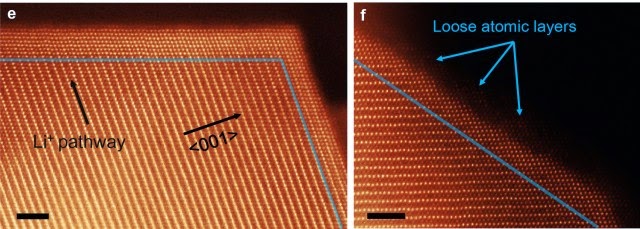
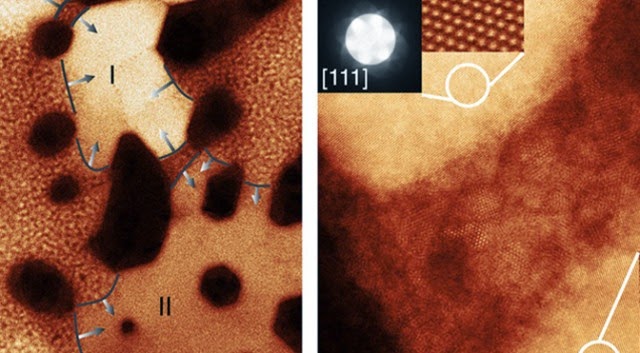
The key to unraveling this mystery was to make careful, direct observations of the cathode and anode material used in modern batteries. Scientists had already pinpointed these components as the site of age-related battery erosion, but the specific mechanism was unclear. The team from Brookhaven National Laboratory used a very sensitive transmission electron microscope (TEM) to observe the changes in high-quality nickel-oxide anodes as they were repeatedly charged and discharged.
The experiment showed that as lithium ions pass through cathodes and anodes, they slowly become stuck within the ion channels due to reactions with nickel oxide to produce small crystals (salt buildup, essentially). These crystals alter the structure of the battery and cause other ions to move less efficiently, thus lowering the usable capacity. Surprisingly, the degradation observed by the Brookhaven team didn’t seem to follow any discernible pattern, at least at first.
The ultimate cause of a lithium-ion battery’s imperfections is that, well, its components aren’t perfect. The anode and cathode materials, no matter how carefully constructed, have minuscule imperfections that act as a nucleation site for crystal formation. It’s a bit like heating water in a totally smooth container versus one with surface disruptions. The bubbles need some sort of irregularity on which to form, and it’s the same with the nanocrystals in batteries. The team refers to this as the chink in the anode’s armor. If there is a place for the crystals to form, they will.
The second study from the Department of Energy’s National Renewable Energy Laboratory looked at the effects of charge speed and capacity on batteries, but focused more on the cathode. This research showed that the race to make higher density batteries could actually be hurting longevity — the bigger the battery and faster it charges, the fewer cycles you get before the nanocrystallization begins to affect it.
So if we can’t simply prevent these nanocrystals from forming, is there a way to reverse the process or at least slow it? It may be possible to treat battery components with a type of atomic deposition to fill in as many of the tiny gaps and imperfections as possible with nanoparticles. This would at least slow the formation of blockages in the ion channels. Even if that doesn’t solve the problem entirely, it could allow engineers to continue ramping up energy density without sacrificing durability. Careful examination of the structure of these crystals could even lead to ways of breaking them apart to revitalize old batteries.
This research might end up being more vital than efforts to boost the capacity of batteries to power faster hardware — in many cases the lifetime of a product is determined by how many cycles the battery can take. This is becoming even more important as companies increasingly push non-removable batteries in laptops, smartphones, and tablets, yet again reminding us that we truly are slaves of electricity.